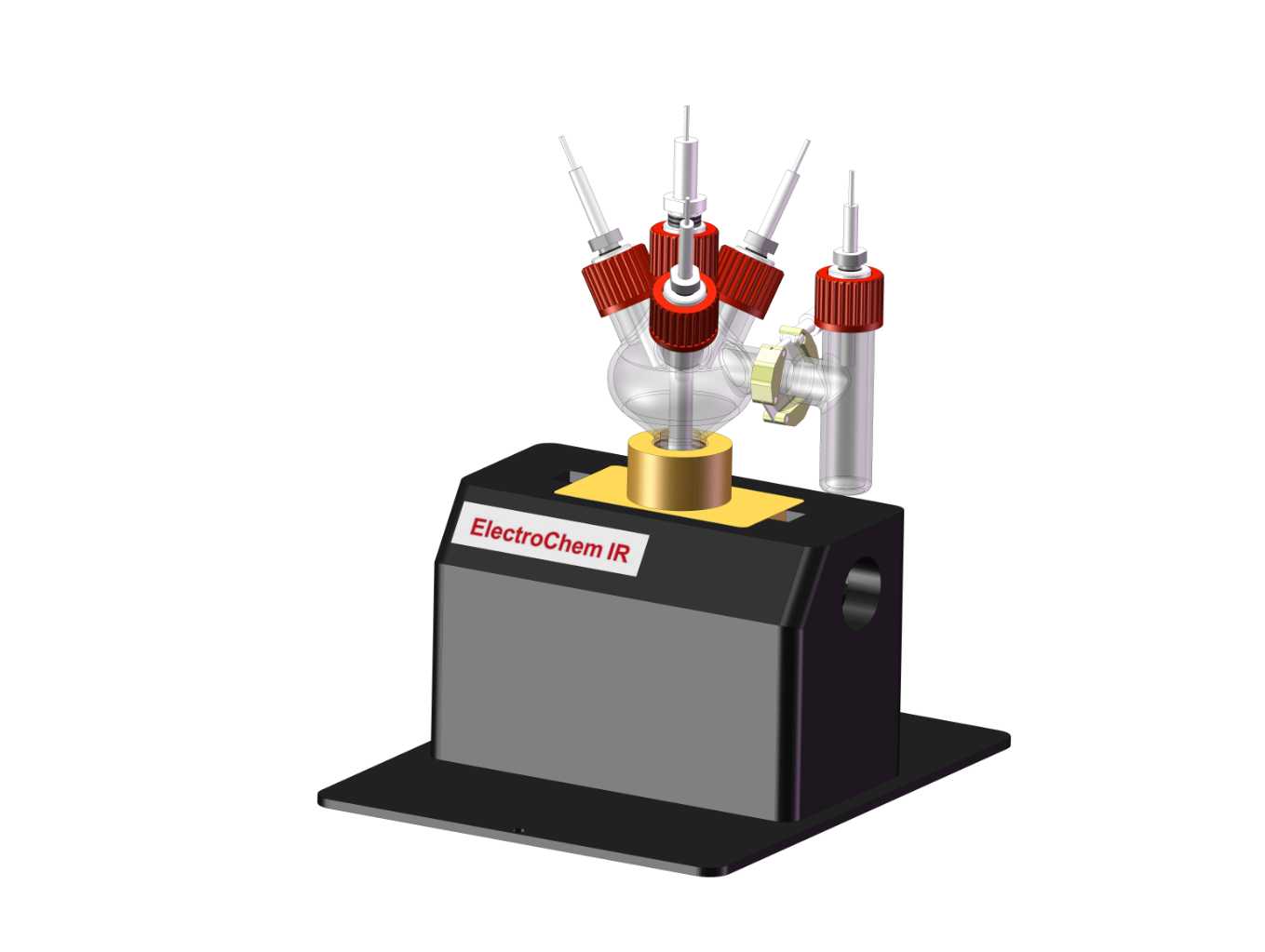
Classic DEMS Electrochemical Cell
Classic DEMS Electrochemical Cell
Differential Electrochemical Mass Spectrometry (DEMS) is an in-situ electrochemical method that provides qualitative and quantitative information about interfacial processes by detecting volatile products. It has become an indispensable tool in the study of electrochemical reaction mechanisms. The DEMS system combines an electrochemical reaction setup with a mass spectrometer, allowing the volatile products generated during the electrochemical reaction to enter the vacuum system of the mass spectrometer through a hydrophobic permeable membrane interface. The mass spectrometer then records the variation of ion current with time for different mass-to-charge ratio ions. Among the commonly used electrochemical techniques for studying reaction mechanisms, cyclic voltammetry (CV) stands out as a versatile method that provides rich electrochemical information. Consequently, CV is frequently employed in DEMS studies. During an electrochemical study using DEMS, the ion current signals of volatile products generated during the CV scan are monitored by the mass spectrometer as a function of time. These signals are then transformed from the time axis to the potential axis to obtain a graph of ion current as a function of potential (MSCV), offering a more comprehensive and in-depth understanding for the investigation of electrocatalytic reaction mechanisms.
Main Features:
1. Stationary or flow-through system.
2. Small electrolyte volume (<1 mL), particularly suitable for isotope labeling experiments.
3. The membrane interface, composed of a porous working electrode and a hydrophobic permeable membrane, directly connects to the mass spectrometer chamber.
4. High collection efficiency (>95%) and high sensitivity.
5. Fast response time (<1 s).
6. Unique gold plating technology.
7. Applicable to powder catalysts or carbon paper-supported catalysts.
8. Suitable for photocatalytic or photoelectrocatalytic reactions.
Specific Applications Include:
1. Instantaneous detection of gas-phase products (CO, CH4, C2H4, CH3OH, etc.) in CO2 electrocatalytic reduction and determination of relative Faradaic efficiency.
2. In-situ detection of intermediate products or final products (NO, N2O, NH2OH, NH3, N2, etc.) in nitrate electrocatalytic reduction.
3. Isotope labeling (18O) in water electrolysis OER for confirmation of LOMor AEM reaction mechanisms.
4. Instantaneous detection and current efficiency calculation of intermediate products or final products (HCHO, HCOOH, CO, etc.) in methanol electrooxidation reactions.
5. Hydrogen isotope labeling and analysis of hydrogen evolution reaction (HER) mechanisms.
6. Evaluation of carbon material stability (detection of CO and CO2 under high potential).
7. Other applications including photocatalysis, photoelectrocatalysis, oxygen reduction, hydrogen evolution, chlorine evolution, organic electrosynthesis, etc.
Application Cases:
1.Detection of intermediates in nitrate electrochemical reduction.
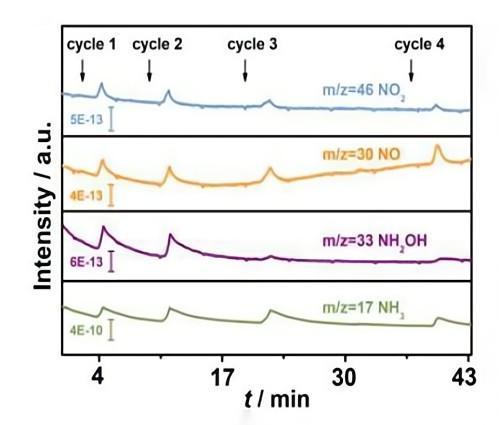
Angew. Chem. Int. Ed. 10.1002/anie.201915992
2. Isotope labeling (18O) in water electrolysis OER for the confirmation of LOM or AEM reaction mechanisms.
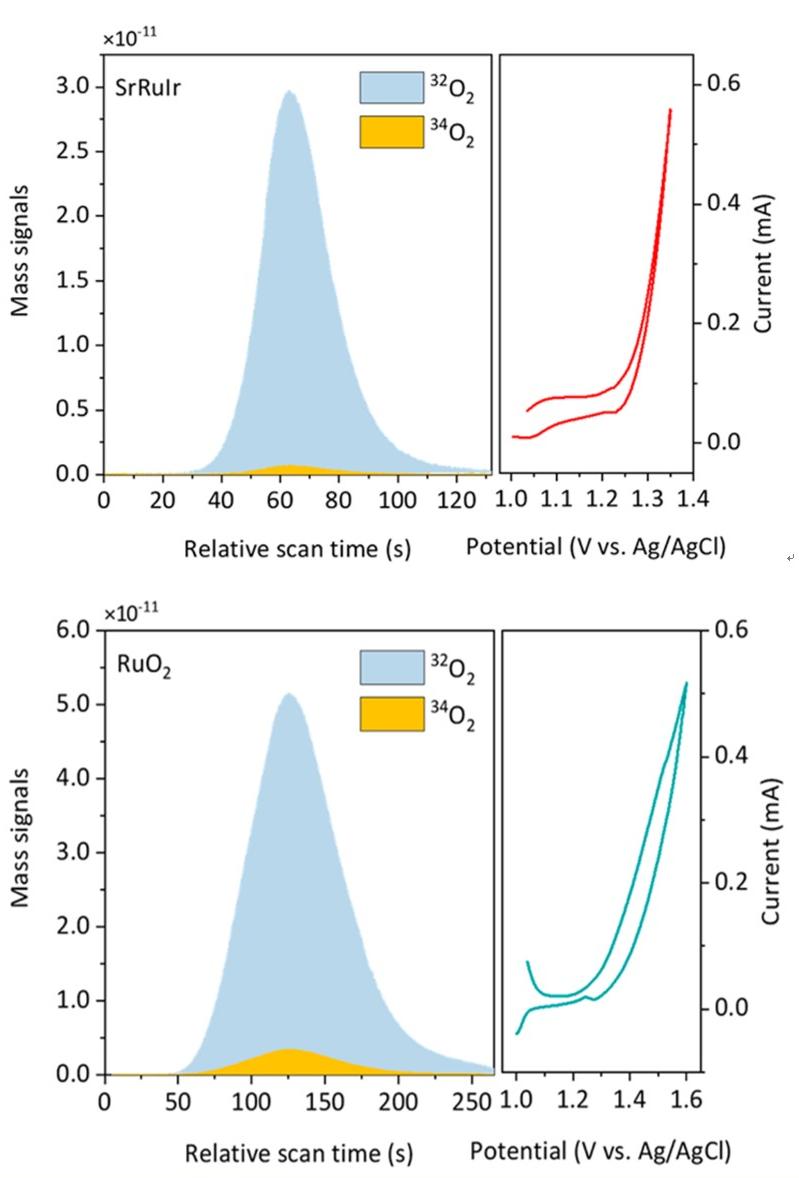
J. Am. Chem. Soc. 2021, 143, 17, 6482-6490
3. Methanol electrooxidation reaction.
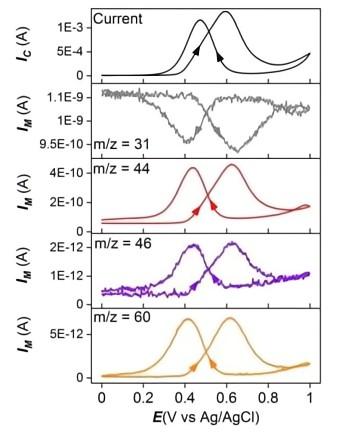
Journal of Power Sources 509 (2021) 230397
4. Hydrogen isotope labeling and analysis of hydrogen evolution reaction (HER) mechanisms.
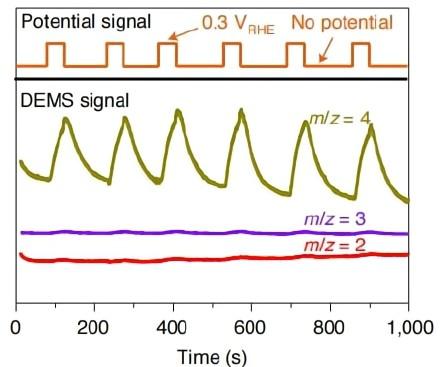
Nature catalysis,2022,5,66-73
5. CO2 electrochemical
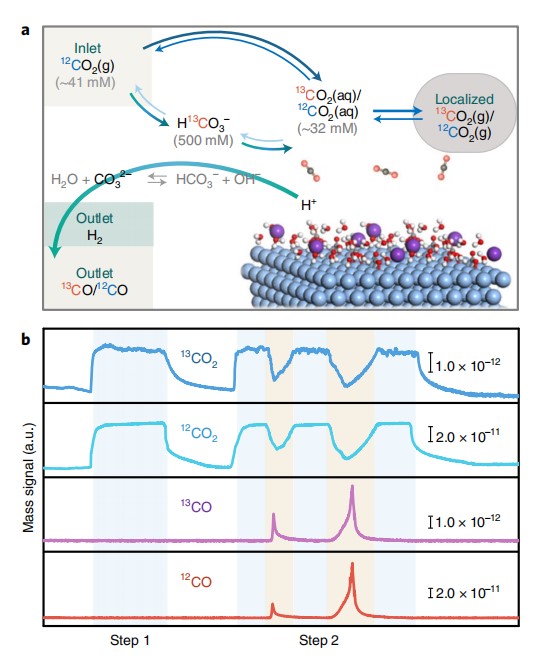
ACS catal. 2019,9,1383-1388
List of Some Customer Papers:
Nature Catalysis. 2022, 5, 66-73
Nature Catalysis. 2021, 4, 1012-1023
J. Am. Chem. Soc. 2021, 143, 6482-6490
J. Am. Chem. Soc. 2019, 141, 9444-9447
Angew. Chem. Int. Ed. 2020, 59, 5350-5354
Angew. Chem. Int. Ed. 2019, 131, 4670-4674
Angew. Chem. Int. Ed. 2021, 60, 7297-7307
Angew. Chem. Int. Ed. 2021, 60, 22933-22939
Angew. Chem. Int. Ed. 2021, 60, 26177-26183
Angew. Chem. Int. Ed. 2022, e202204541
Joule. 2021, 5, 2164-2176
Nat. Commun. 2022, 13, 2191
Nat. Commun. 2021, 12, 2164
Adv. Mater. 2020, 32, 2002297
Adv. Energy Mater. 2020, 10, 2001289
Appl. Catal. B. 2021, 280, 119393
ACS Energy Letters. 2022, 7, 1187-1194
ACS Energy Letters. 2022, 7, 284-291
Chem. Eng.J. 2022, 435, 134969
Chem. Eng.J. 2022, 433, 133495
Environ. Sci. Technol. 2022, 56, 614-623
ACS Catal. 2021,11, 840-848
ACS Catal. 2019, 9, 4699-4705
Nano Energy. 2021, 86, 106088
NanoEnergy. 2019, 60, 43-51
ACS Catal. 2021, 11, 14032-14037
ACS Catal. 2020, 10, 3533-3540
ACS Appl. Mater. Interfaces. 2022, 14, 12257-12263
J. Mater. Chem. A. 2021, 9, 239-243
Cell Reports Physical Science. 2021, 2, 100378
J. Mater. Chem. A. 2021, 9, 9010-9017
Journal of Catalysis. 2021, 397, 128-136
Journal of Power Sources. 2021, 509, 230397
Science China Chemistry. 2020, 63, 1469-1476
Adv. Sustainable Syst. 2020, 4, 2000227
Science China Chemistry.2021, 64, 1493-1497
J. Colloid Interface Sci. 2022, 614, 405-414
Angew. Chem. Int. Ed. 2022, 61, e20211563
Nat. Commun. 2022, 13, 2577
J. Mater. Chem. A. 2022, 10, 6448–6453
J. Mater. Chem. A. 2021, 9, 14741–14751
ACS Sustainable Chem. Eng. 2022, 10, 5958–5965
J. Mater. Chem. A. 2022, 10, 5430-5441
Appl. Catal. B. 2022, 301, 120829
Adv. Mater. 2020, 2202523
Adv. Mater. 2020, 2202874
ACS Catal. 2022, 12, 14, 8658–8666
Energy Environ. Sci. 2022,15, 3912-3922
Adv. Mater. 2022, 2209307
Angew. Chem. Int. Ed. 2023, e202217071
ACS Nano. 2022, 16, 6, 9095–9104
Angew. Chem. Int. Ed. 2022, 61, e202212341
J. Am. Chem. Soc. 2022, 144, 35, 16006–16011
Adv. Energy Mater. 2022, 12, 2103960
Nature Energy. 7, 978–988 (2022)
Energy Environ. Sci. 2022, 15, 4175
Nat. Commun. (2022) 13:7958